Embark on a scientific adventure with the potato osmosis lab answer key, your trusty guide to unraveling the mysteries of osmosis and its profound impact on living cells. This comprehensive resource empowers you to delve into the intricacies of potato osmosis, deciphering the intricate dance between water molecules and cell membranes.
As we embark on this exploration, we will dissect the fundamentals of osmosis, unravel the intricacies of the potato osmosis lab experiment, and delve into the fascinating implications of osmosis for biological systems. Prepare to be captivated as we unlock the secrets of cell behavior and uncover the practical applications of osmosis in various fields.
Potato Osmosis
Osmosis is a crucial process in biology that involves the movement of water molecules across a semipermeable membrane. In the context of potato osmosis, this membrane is the cell wall of the potato cells. The experiment is designed to demonstrate the principles of osmosis by observing changes in potato cells when they are placed in different concentrations of salt solutions.
In the potato osmosis lab, potato slices are submerged in solutions with varying salt concentrations. These solutions can be classified as hypertonic, hypotonic, or isotonic, depending on their solute concentration relative to the potato cells.
Hypotonic Solution
When potato slices are placed in a hypotonic solution, the water molecules move from the solution into the potato cells, causing the cells to swell and become turgid. This is because the concentration of water molecules is higher in the solution than inside the potato cells, creating a net movement of water into the cells.
Materials and Procedure
To conduct this experiment, you’ll need the following materials:
- Potatoes
- Potato corer or knife
- Salt
- Sugar
- Water
- Graduated cylinders
- Beakers
- Stopwatch or timer
- Data table
Here’s a step-by-step procedure for conducting the experiment:
Creating a Data Table
Before starting the experiment, create a data table to record your observations. The table should include the following columns:
| Potato Core | Solution | Initial Mass (g) | Final Mass (g) | Change in Mass (g) | Percentage Change (%) |
|---|
Data Analysis and Interpretation
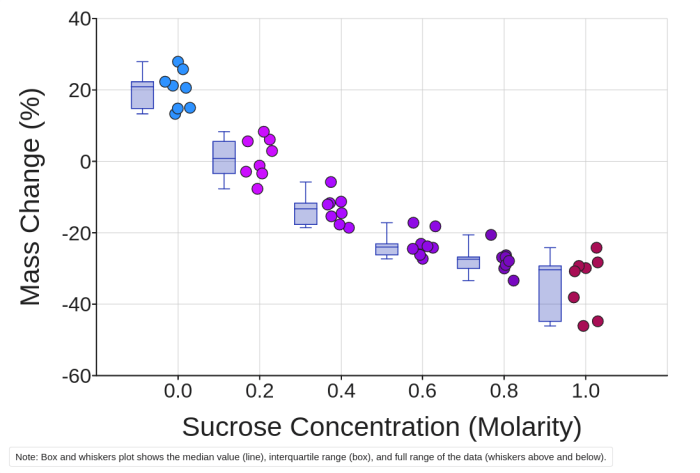
To analyze the data, first calculate the percentage change in mass for each potato slice. The percentage change is calculated using the formula: “` % Change in Mass = ((Final Mass – Initial Mass) / Initial Mass) – 100 “`
Next, plot a graph with the percentage change in mass on the y-axis and the solute concentration on the x-axis. The graph should show a general trend of increasing percentage change in mass with increasing solute concentration.
Expected Trends and Patterns
The expected trends and patterns in the data are as follows:
- Potato slices placed in distilled water will gain weight because water moves into the cells by osmosis.
- Potato slices placed in a hypertonic solution will lose weight because water moves out of the cells by osmosis.
- The greater the concentration of the solute in the solution, the greater the percentage change in mass.
Interpreting the Results
The results of the experiment can be interpreted in terms of potato cell behavior. When potato slices are placed in a hypotonic solution, water moves into the cells by osmosis. This causes the cells to swell and the potato slices to gain weight.
When potato slices are placed in a hypertonic solution, water moves out of the cells by osmosis. This causes the cells to shrink and the potato slices to lose weight.
Discussion
The experiment’s findings highlight the critical role of osmosis in biological systems. Osmosis is the net movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. This process plays a vital role in maintaining cell volume, shape, and overall function.
In the potato osmosis experiment, when potato slices were placed in different salt solutions, water moved either into or out of the cells, depending on the concentration of the solution. This movement of water was driven by the difference in water potential between the potato cells and the surrounding solution.
When potato slices were placed in a hypotonic solution (lower salt concentration than the cells), water moved into the cells, causing them to swell and become turgid. Conversely, when potato slices were placed in a hypertonic solution (higher salt concentration than the cells), water moved out of the cells, causing them to shrink and become flaccid.
Implications of Osmosis for Potato Cells and Other Biological Systems
The implications of osmosis for potato cells and other biological systems are far-reaching. In plants, osmosis plays a crucial role in water uptake and transport throughout the plant. It also helps maintain cell turgor, which is essential for plant growth and support.
In animals, osmosis is involved in fluid balance, cell volume regulation, and the absorption and excretion of nutrients.
Understanding osmosis is also essential for comprehending various physiological processes, such as blood pressure regulation, kidney function, and the absorption of nutrients in the digestive tract. Dysregulation of osmosis can lead to a variety of health conditions, including dehydration, edema, and cell damage.
Factors Affecting Osmosis and Cell Behavior
Several factors can affect osmosis and cell behavior, including:
- Solute concentration:The greater the difference in solute concentration between the two solutions, the greater the rate of osmosis.
- Temperature:Osmosis occurs more rapidly at higher temperatures because the water molecules have more kinetic energy.
- Surface area:The larger the surface area of the membrane, the faster the rate of osmosis.
- Membrane permeability:The permeability of the membrane to water molecules also affects the rate of osmosis. Membranes that are more permeable to water allow osmosis to occur more quickly.
Applications
Osmosis finds widespread applications in various fields, including food processing, medicine, and agriculture. Its ability to selectively transport water molecules across a semipermeable membrane makes it a valuable tool in numerous industries.
In food processing, osmosis is employed to preserve and enhance the quality of food products. It is used in techniques such as dehydration, which removes water from fruits and vegetables to extend their shelf life. Osmosis also plays a role in the production of cheese and yogurt, where it helps in removing excess water and concentrating the flavor.
Medicine
In the medical field, osmosis is utilized in a variety of applications. One notable example is hemodialysis, a procedure that uses osmosis to remove waste products from the blood of patients with kidney failure. Osmosis is also employed in drug delivery systems, where it enables controlled release of medications into the body.
Agriculture, Potato osmosis lab answer key
In agriculture, osmosis plays a crucial role in plant growth and development. It governs the uptake of water and nutrients by plant roots, influencing their overall health and productivity. Understanding osmosis helps farmers optimize irrigation practices and improve crop yields.
If you’re struggling with the potato osmosis lab answer key, don’t fret. Check out our comprehensive guide for all the answers you need. And if you’re looking for a fun way to test your brain, try our pains in the neck crossword . But don’t worry, we’ll still be here to help you with the potato osmosis lab answer key when you’re ready.
Further Research: Potato Osmosis Lab Answer Key
The realm of potato osmosis holds a treasure trove of untapped potential for scientific exploration. By venturing beyond the confines of this lab, researchers can unravel new frontiers of knowledge, illuminating the intricate workings of osmosis and its profound impact on plant cells.
One promising avenue for further research lies in investigating the role of specific solutes in osmosis. Different solutes exhibit varying abilities to penetrate cell membranes, and understanding these differences can shed light on the selective permeability of plant cells. By manipulating the solute concentration and composition, researchers can delve into the mechanisms that govern water movement across membranes.
The Influence of Temperature on Osmosis
Temperature plays a crucial role in biological processes, and its impact on osmosis is no exception. Exploring the effects of temperature on the rate and extent of osmosis can provide valuable insights into the adaptability of plant cells to changing environmental conditions.
By exposing potato slices to a range of temperatures, researchers can determine the optimal conditions for osmosis and uncover the underlying mechanisms responsible for temperature-dependent changes.
The Application of Osmosis in Biotechnology
The principles of osmosis extend beyond the laboratory and find practical applications in various fields, including biotechnology. By harnessing the power of osmosis, scientists can develop innovative technologies for water purification, drug delivery, and biomaterial engineering. Further research in this area can lead to advancements in healthcare, environmental sustainability, and the development of novel materials.
The Importance of Ongoing Research
Ongoing research in potato osmosis is paramount for advancing our scientific understanding and unlocking its potential applications. By continuously pushing the boundaries of knowledge, researchers can uncover new insights into the fundamental processes that govern life on Earth. Moreover, this research contributes to the collective body of scientific knowledge, providing a foundation for future discoveries and technological advancements.
FAQ Resource
What is the purpose of the potato osmosis lab?
The potato osmosis lab demonstrates the process of osmosis and its impact on plant cells. It helps students understand how water moves across a semipermeable membrane and how this affects cell size and shape.
What factors affect the rate of osmosis?
The rate of osmosis is influenced by several factors, including the concentration gradient of the solutions, the surface area of the membrane, the temperature, and the presence of solutes.
What are the practical applications of osmosis?
Osmosis has numerous practical applications, such as in food processing (e.g., making pickles), medicine (e.g., administering intravenous fluids), and agriculture (e.g., regulating water uptake in plants).