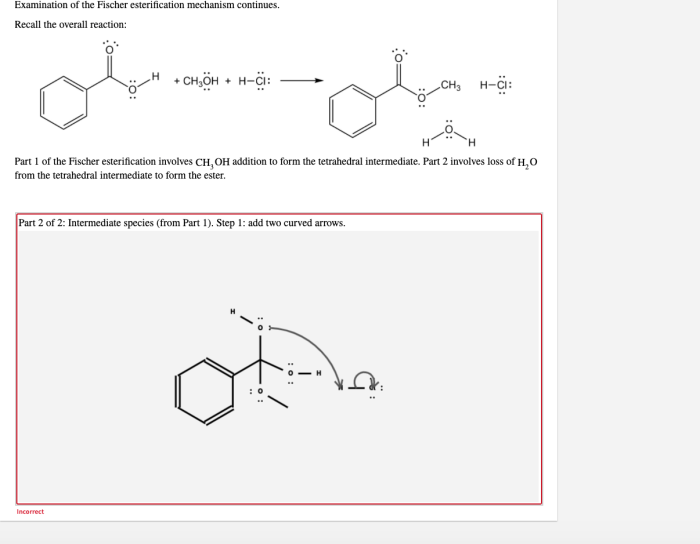
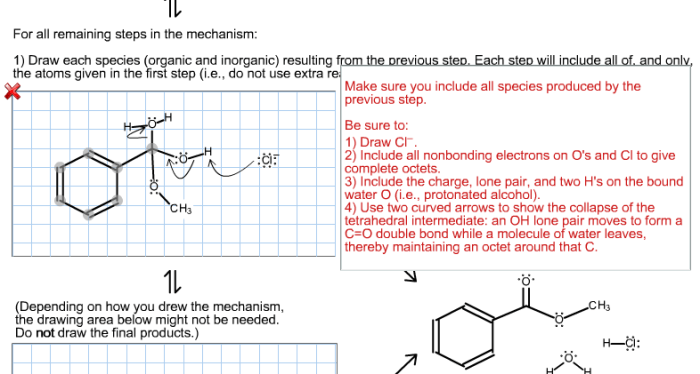
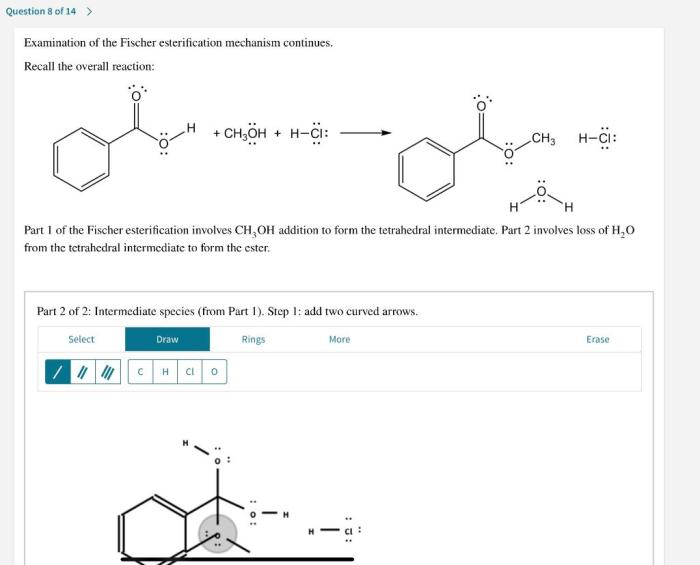
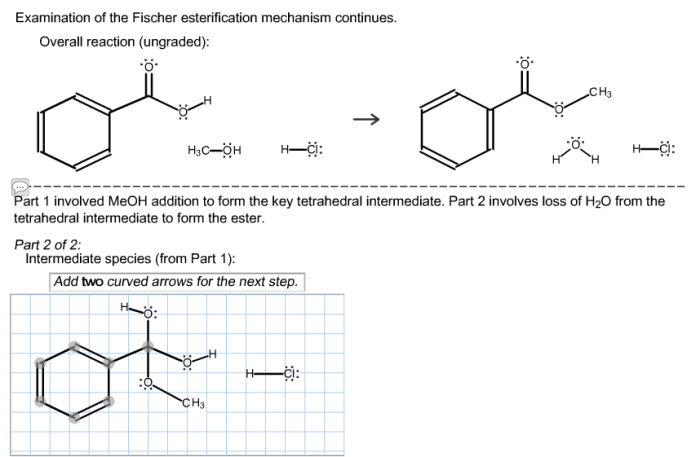
Examination of the fischer esterification mechanism continues. – Examination of the Fischer esterification mechanism continues, setting the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset.
The Fischer esterification reaction holds immense significance in organic chemistry, providing a versatile method for the synthesis of esters. Over the years, the understanding of its mechanism has evolved, thanks to the contributions of brilliant scientists and the advent of advanced techniques.
Historical Overview of Fischer Esterification
Fischer esterification, a seminal reaction in organic chemistry, enables the synthesis of esters from carboxylic acids and alcohols. This reaction holds immense significance in the production of diverse organic compounds, including fragrances, flavors, and pharmaceuticals.
The historical journey of Fischer esterification began in 1894 when Emil Fischer, a renowned German chemist, elucidated the fundamental principles of this reaction. Over the years, numerous scientists have contributed to unraveling the intricacies of its mechanism, leading to a comprehensive understanding of its underlying processes.
Detailed Examination of the Reaction Mechanism
Fischer esterification involves a series of sequential steps:
- Protonation of the carboxylic acid by a strong acid catalyst, such as sulfuric acid.
- Nucleophilic attack by the alcohol on the protonated carboxylic acid, forming a tetrahedral intermediate.
- Collapse of the tetrahedral intermediate, expelling a water molecule and generating the ester product.
The rate and equilibrium of Fischer esterification are influenced by several factors, including temperature, solvent, and catalyst concentration.
Applications of Fischer Esterification in Organic Synthesis
Fischer esterification finds widespread application in organic synthesis:
- Production of esters, amides, and anhydrides.
- Synthesis of fragrances, flavors, and pharmaceuticals.
- Preparation of biodiesel from vegetable oils.
Compared to other esterification methods, Fischer esterification offers advantages such as mild reaction conditions and compatibility with a wide range of substrates.
Recent Advances and Future Directions in Fischer Esterification Research, Examination of the fischer esterification mechanism continues.
Recent research efforts have focused on:
- Developing greener and more efficient catalysts.
- Exploring applications in biocatalysis and green chemistry.
- Utilizing computational chemistry and isotopic labeling to gain deeper insights into the reaction mechanism.
Further research is necessary to improve the selectivity and efficiency of Fischer esterification, expanding its utility in various fields of chemistry.
Common Queries: Examination Of The Fischer Esterification Mechanism Continues.
What are the key steps involved in the Fischer esterification reaction?
The Fischer esterification reaction involves the reaction of an acid and an alcohol in the presence of a catalyst, typically an acid. The reaction proceeds through the formation of a tetrahedral intermediate, which subsequently collapses to form the ester product.
What factors affect the rate and equilibrium of the Fischer esterification reaction?
The rate and equilibrium of the Fischer esterification reaction are influenced by several factors, including temperature, solvent, and catalyst concentration. Higher temperatures favor the forward reaction, while polar solvents promote the formation of the tetrahedral intermediate. Catalyst concentration also plays a crucial role, with higher concentrations leading to faster reaction rates.